qCART™
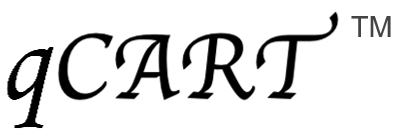
Overcomes the Challenges of CAR T Cell Therapy
Chimeric antigen receptor T (CAR T) cell therapy represents a major breakthrough in cancer therapy and has produced remarkable clinical responses in certain hematological cancer. However, several obstacles must be overcome before CAR T cell therapy can be widely applied for many cancers. These include (1) toxicity due to on-target/off-tumor activities and/or cytokine release syndrome (CRS); (2) disease relapses due to: (i) antigen escape and/or antigen heterogeneity of solid tumor, resulting in outgrowth of non-targeted tumor cells and/or (ii) a lack of persistence of CAR T cells; (3) lack of potency for solid tumors; and (4) high costs associated with CAR T cell manufacturing.
High levels of TSCM cells in the CAR T cell population can lead to better clinical outcomes. TSCM cells exhibit stem-like properties with high capacity for self-renewal, whcih contributes to their long-term persistence. Furthermore, TSCM cells have the highest therapeutic efficacy due to enhanced metabolic fitness and low level of senescence and exhaustion (Figure 1).
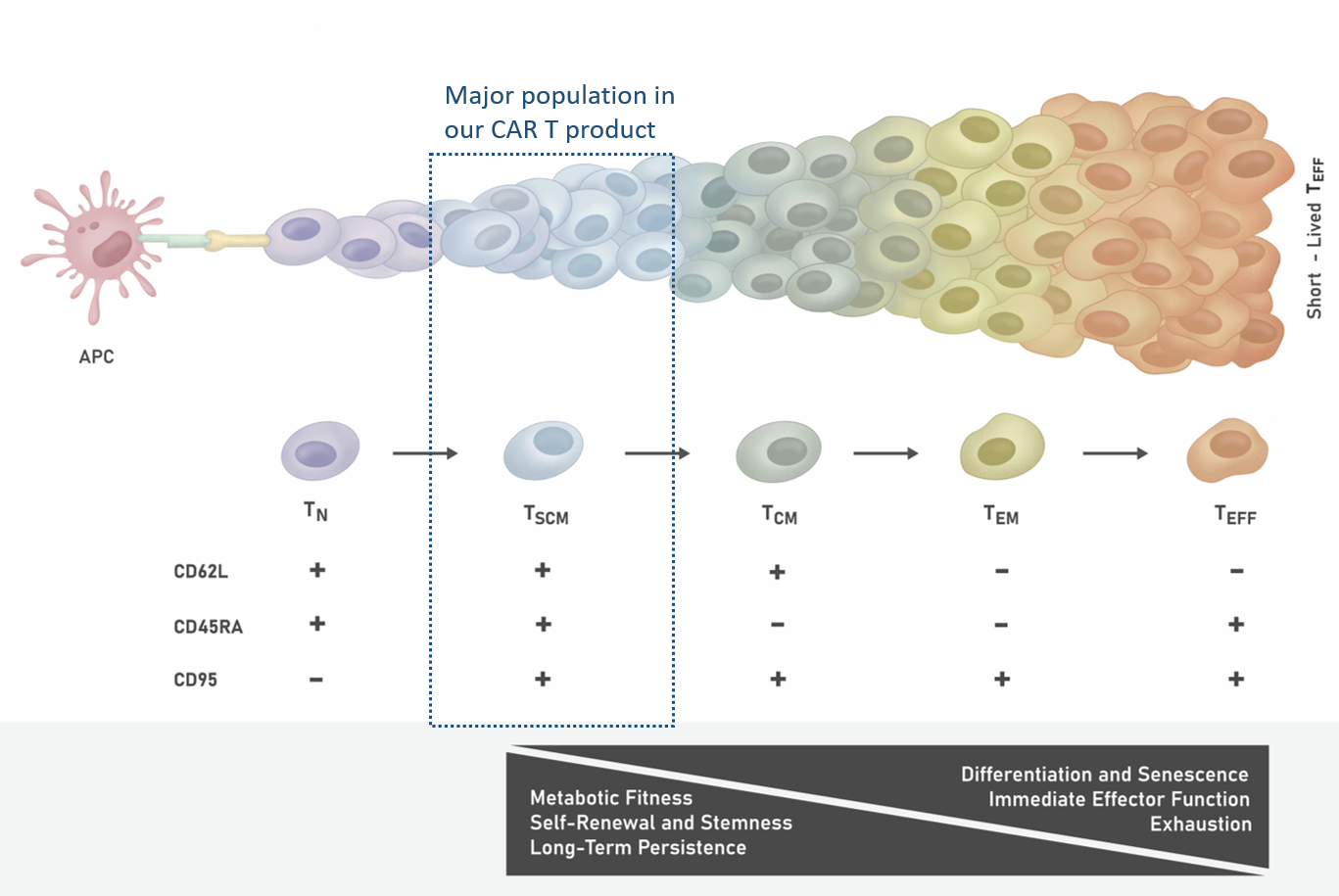
Figure 1. T Cell Differentiation Stages Upon Antigen Stimulation
Following the activation of TN cells by tumor associated antigens (presented on antigen presenting cells, or APC), long-lived TSCM and TCM are produced. These self-renewing T cells in turn give rise to proliferating populations of shorter-lived TEM and TEFF cells. TSCM cells are thought to be the most therapeutically efficacious T cell population.
CAR T cell therapy faces several challenges in the context of solid tumor, including (1) lack of CAR T cell trafficking to the tumor; (2) antigen heterogeneity of solid tumors; and (3) immunosuppressive tumor microenvironment (TME) which can adversely affect T cell fitness (e.g. differentiation, exhaustion, senescence, and survival). A technological breakthrough needs to be developed to overcome these hurdles.
Viral vector has been widely used for the development of CAR T cell therapy due to its high efficiency in gene delivery and integration, resulting in stable long-term gene expression. However, CAR T cells generated virally have several limitations. These include (1) virus-associated safety concerns; (2) limited payload capacity of viral vectors; (3) low expansion capacity and low percentage of CAR⁺ T cells following transduction; (4) low CAR T cell persistence; and (5) high cost of manufacturing virally-produced CAR T cells.
A virus-free system can overcome most if not all of these hurdles, but electroporation-associated damages in virus-free systems present a critical challenge. In order for a virus-free system to be effective, we need: (1) a robust gene delivery system; (2) an effective system for integrating multiple genes into CAR T cells; and (3) a robust cell expansion system for producing clinical-scaled CAR T cells.
GenomeFrontier has developed Quantum CART (qCART™), a virus-free Quantum Engine™ for developing CAR T cell therapy that synergistically integrates four platforms:
(1) G-Tailor™: a rapid multiplex gene design, construction, and screening system for designing CAR T cells with (i) the ability to target to multiple tumor antigens; (ii) modulators for efficient CAR T cell trafficking and TME resistance; and (iii) a safety control to terminate treatment as needed.
(2) Quantum Nufect™: a robust gene delivery buffer system for introducing therapeutic genes into T cells, resulting in (i) reliable CAR T cell production while (ii) preserving high cell viability.
(3) Quantum pBac™: a virus-free vector system with (i) a large payload gene integration capacity and (ii) high preference for TSCM genome integration.
(4) iCellar™: a robust cell expansion system for producing clinical-scaled CAR T cells with (i) high percentage of CAR⁺ TSCM cells and (ii) enhanced fitness.
Collectively, qCART™ enables timely (~10 days) and cost-effective manufacturing of clinical-scaled CAR T cells (1-3.5x109/L) (Table 1, Figure 2 and Figure 3).
Virus-free qCART™ robustly produces clinical-scaled CAR T cells comprised of high percentage of CAR⁺ TSCM cells (Table 1) capable of mediating high tumor cytolytic activities (Figure 2).
Table 1. Direct comparison of CAR T production by perfusion or fed batch
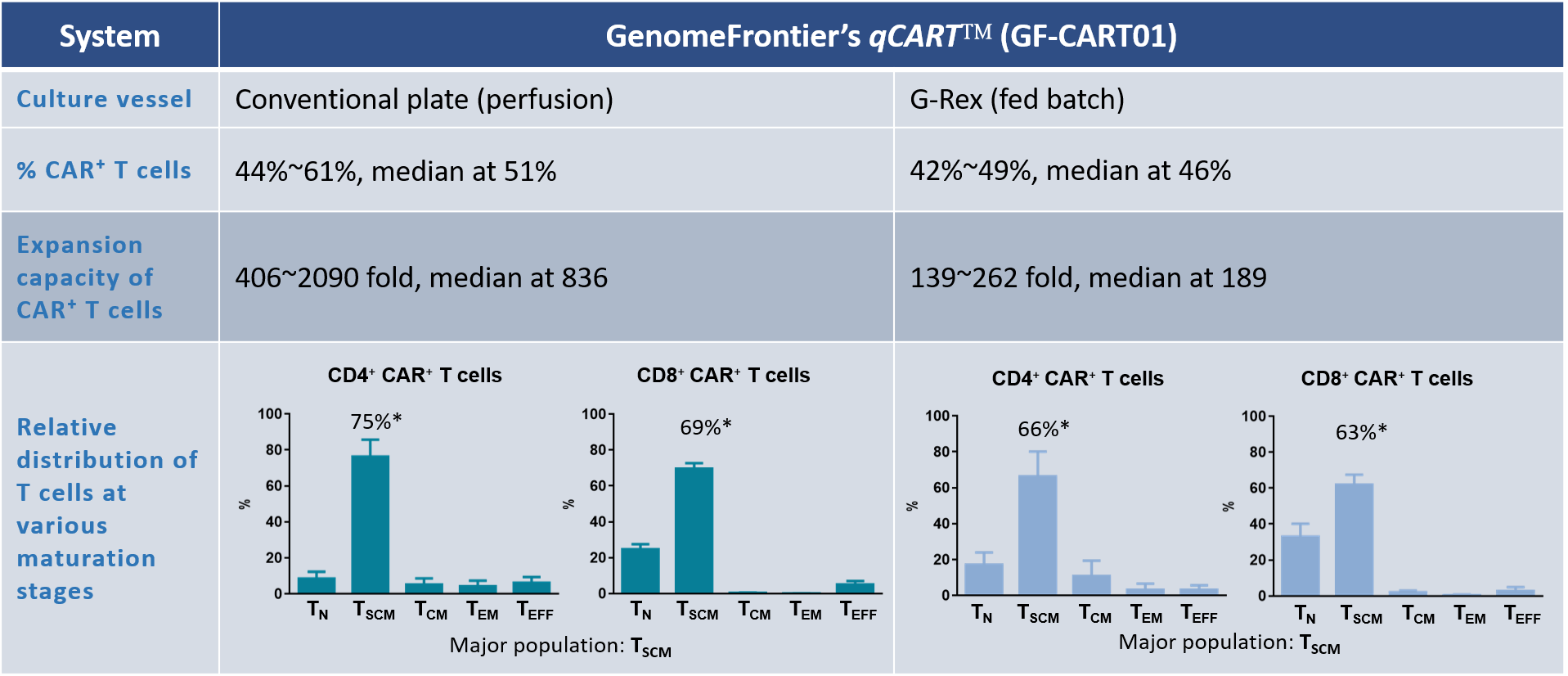
Product characterization of GF-CART01
CAR⁺ T cells were cultured in either the conventional plates (perfusion) or G-Rex (fed batch). The percentage of CAR⁺ T cells, expansion fold change, and distribution of CD4⁺ and CD8⁺ CAR⁺ T cell subsets are shown. Data shown are from six healthy donors.
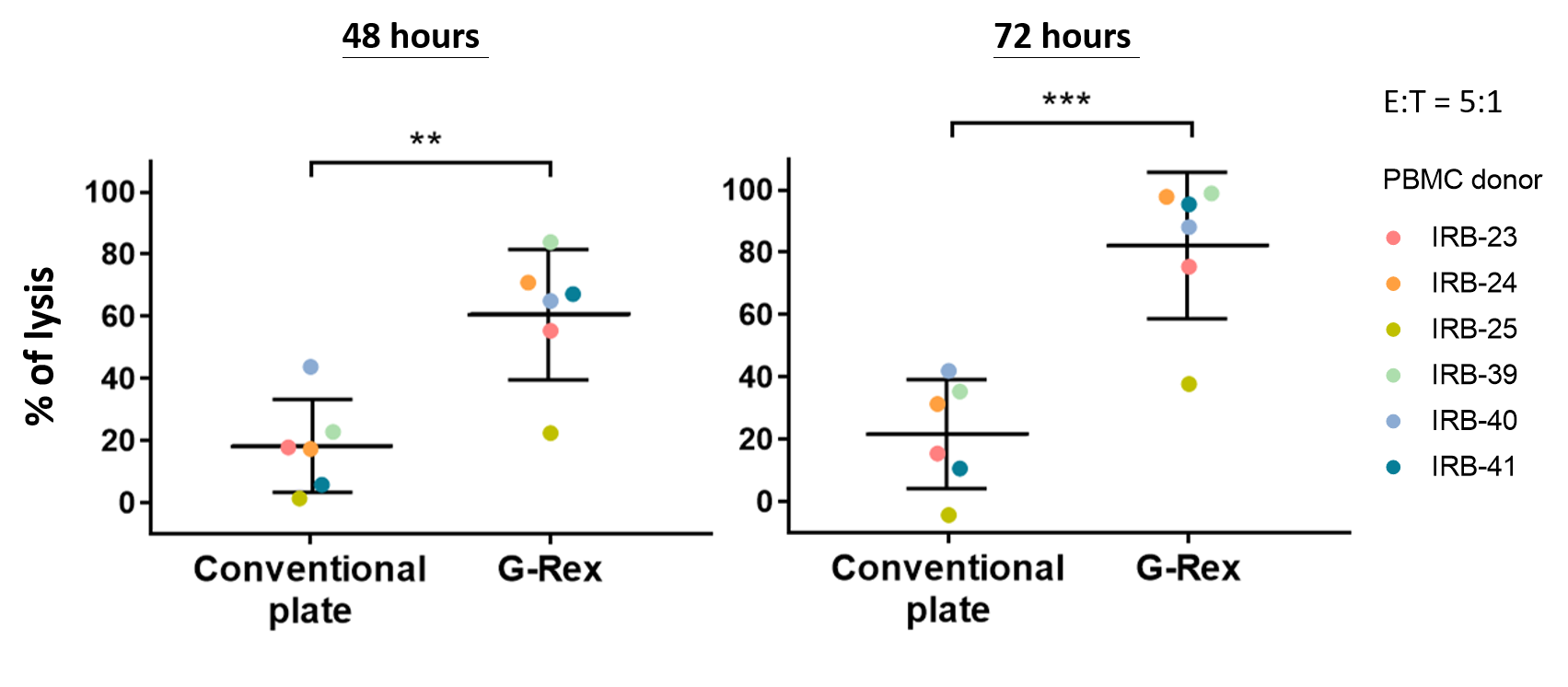
Figure 2. CAR T Cells Produced by the G-Rex System Mediate High Levels of Cytotoxicity
In vitro functional analysis. Thawed CAR T cells were assessed for cytolytic activities using Celigo image cytometry. Cytolytic activities of CAR T cells after 48 or 72 hours of co-culture with Raji-GFP/Luc target cells (E:T ratio of 5:1) are shown. Data shown are from six healthy donors. Horizontal lines represent the mean % lysis of target cells by CAR T cells. **p < 0.01; ***p < 0.001.
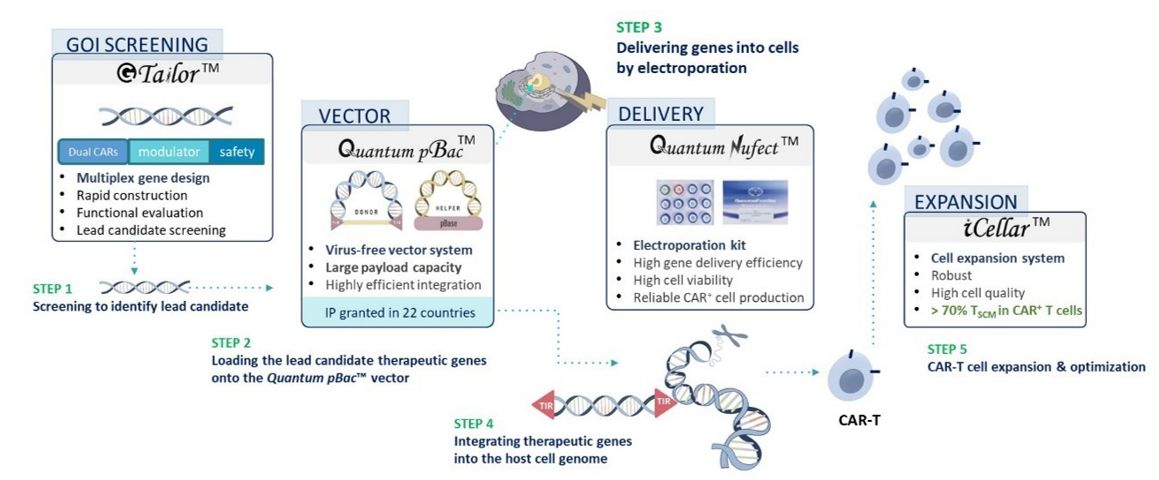
Virus-free qCART™ reliably produces CAR⁺ TSCM cells with high expansion capacity against different targets and with various transgene sizes (Table 2), thereby demonstrating the feasibility of multiplex CAR T construct design.
Table 2. Reliable Performance of CAR T cells against Different Targets and with Various Transgene Sizes