Quantum pBac™

An advanced, virus-free and highly efficient piggyBac-based system with large payload capacity (~100 kb) for optimal therapeutic gene integration
Gene therapy often requires stable, long-term gene expression in cells. Such prolonged expression can be accomplished by engineering cells either ex vivo or in vivo using viral or non-viral vector systems.
Lentiviral/ retroviral vectors are commonly used for gene therapy due to their high efficiencies of gene delivery and their abilities to carry out gene integration, which confer stable and long-term gene expression. However, widespread application of LV/ RV viral vectors have encountered several intrinsic limitation obstacles, including: (1) limited payload capacity, which severely restricts the repertoire of genes that can be integrated; (2) genotoxicity arising from preferential integration of viral vectors in sites near or within active gene loci, which may negatively impact gene expression and function; (3) predisposition of silencing of genes introduced by viral vectors, presumably due to cellular immunity; and (4) immunogenicity of viral vectors. These limitations restrict clinical application of viral vectors. Additionally, viral vector-based gene therapy are expensive to manufacture given the high cost of GMP-compliant viral vectors and the stringent requirements for quality assurance.
Virus-free vector is a potentially a safer alternative due to lower immunogenicity and reduced genotoxicity compared to viral vectors. Moreover, virus-free vector has the capacity to carry larger fragments of genetic material compared to conventional viral vectors. The manufacturing costs of virus-free vectors are also markedly lower than those required for viral vector production. Despite the advantages of virus-free vectors, therapeutic genes in the form of naked DNA are short-lived and lack stable gene expression.
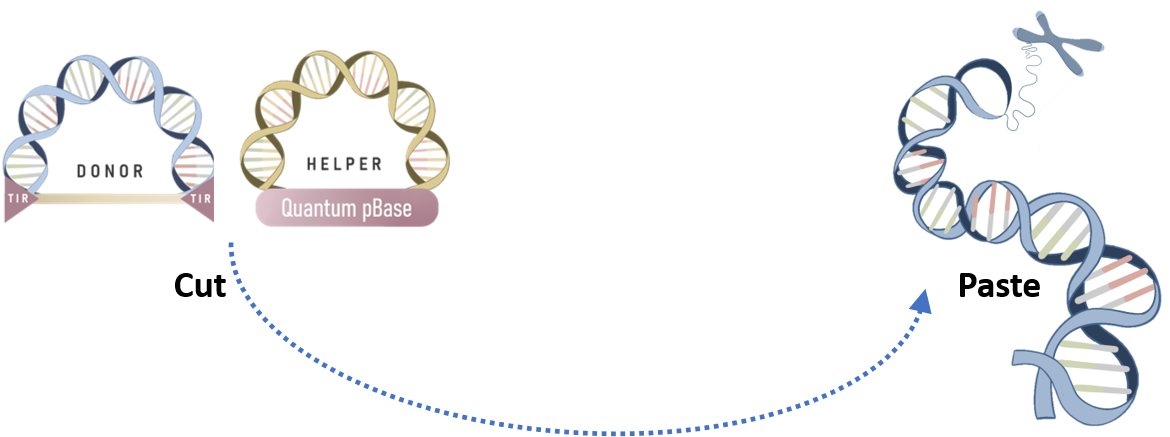
In recent years, virus-free DNA transposon has emerged as a promising vector system for gene therapy, due to its ability to overcome the challenges of gene integration. A commonly used two-plasmid transposon system for mediating gene integration via a simple cut-and-paste mechanism is illustrated below:
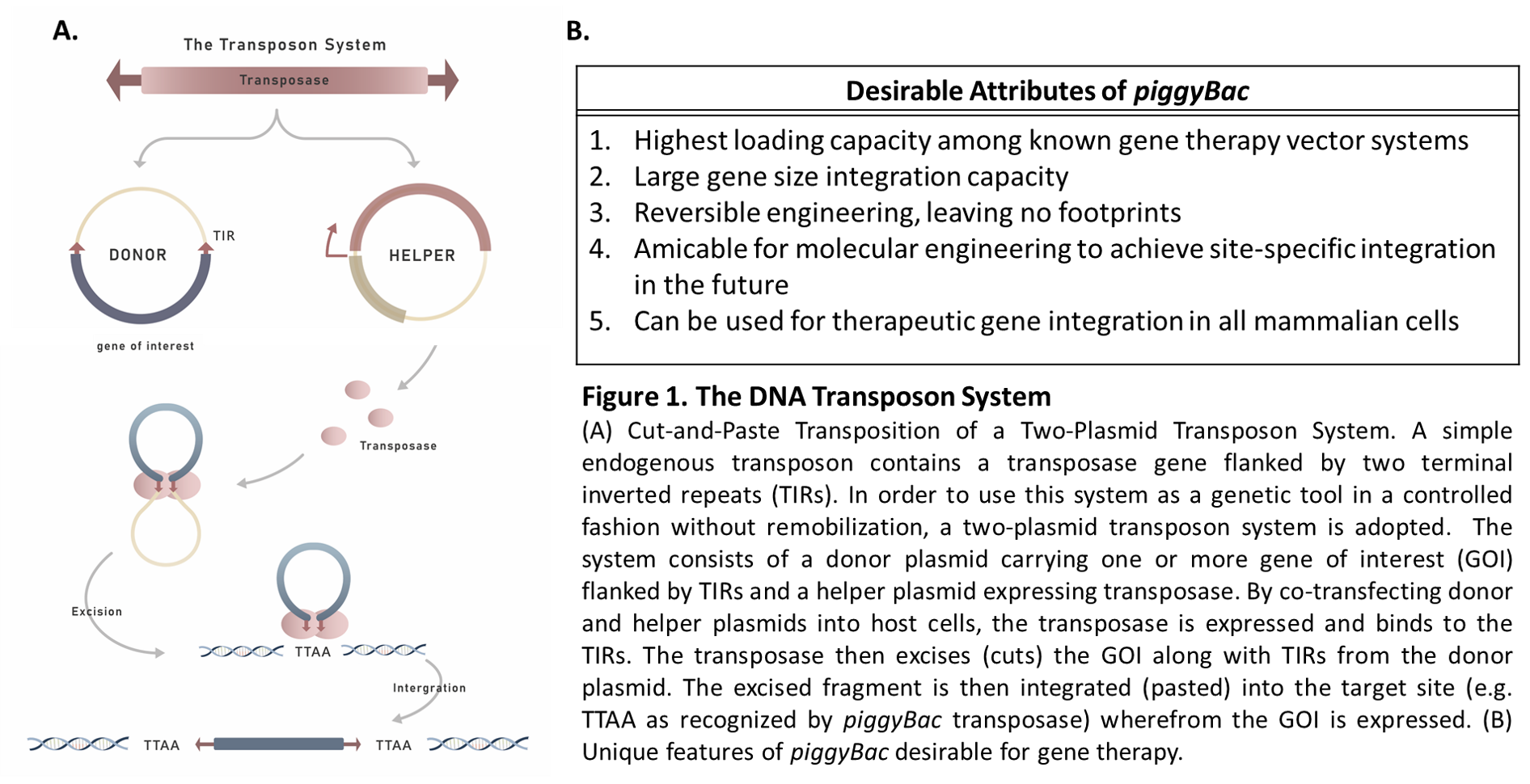
As shown in the Figure 1A above, the most common setup involves the use of a two-plasmid system – one containing the expression cassette flanked by TIRs and the other encoding the transposase. This system is capable of delivering a large payload with minimal loss of efficiency.
As one of the pioneers in the field of virus-free gene therapy, our team was the first to discover that piggyBac, a DNA transposon isolated from cabbage moths, is the most promising DNA transposon system for gene therapy as compared to other DNA transposon systems (Wu S et al., PNAS 2006, 103 (41) 15008-15013). Given the unique desirable features of the piggyBac system for gene therapy (see Figure 1B), advancing the piggyBac transposon system for clinical applications is currently GenomeFrontier’s main focus. Our endeavors have resulted in the development of a potentially safer and more robust piggyBac-based system, named Quantum pBac™ (Figure 3), which holds promise for gene therapy, particularly in CAR T cell therapy (Meir et al., FASEB J 2013, 27 (11) 4429-4443; Meir et al., BMC Biotechnology 2011, 11:28; manuscripts in preparation). The developmental history of Quantum pBac™ in comparison with other therapeutic vectors in the context of CAR T cell therapy arena is illustrated below (Figure 2).
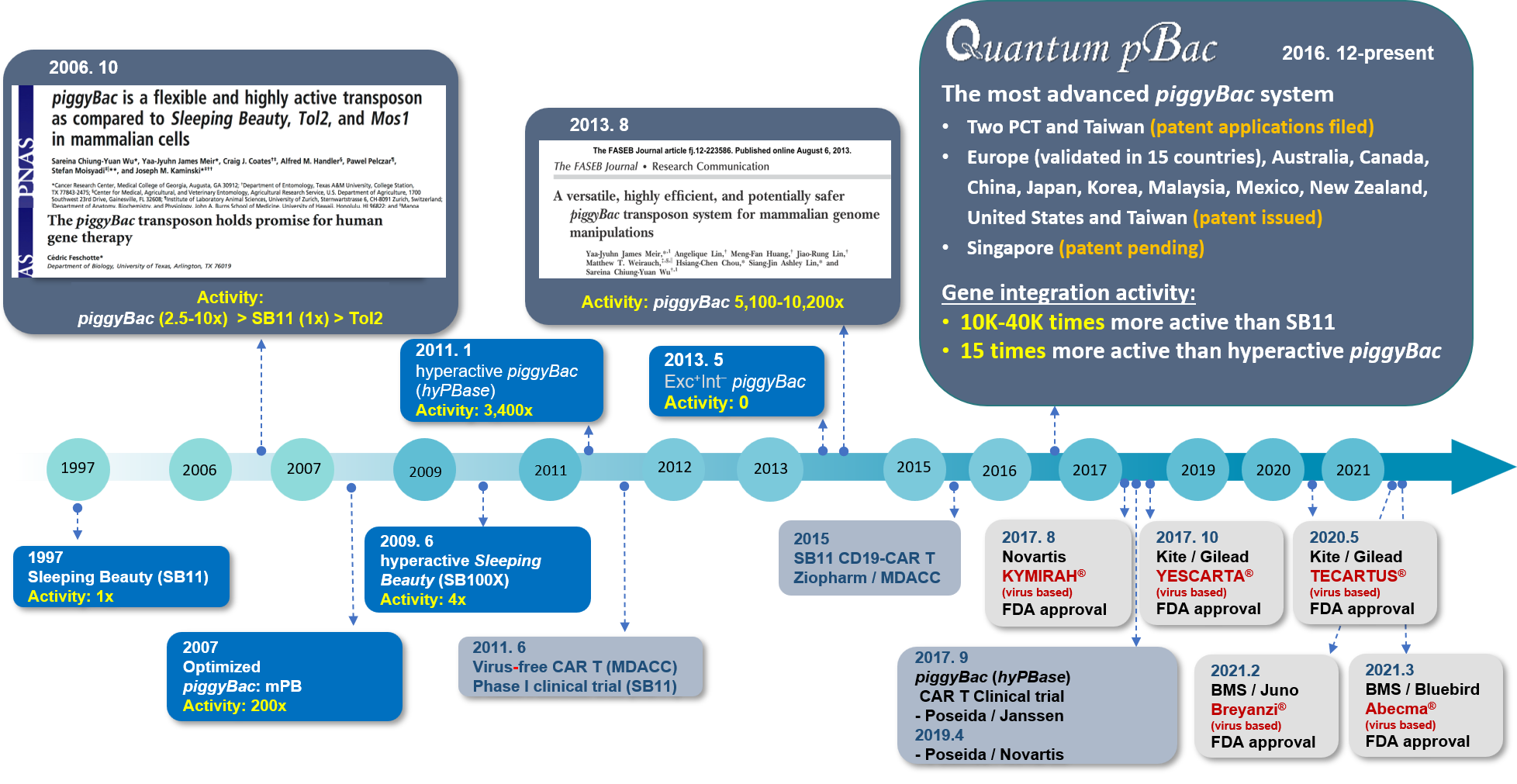
Figure 2. History of Quantum pBac™ Development in the Context of CAR T Cell Therapy Arena
Hyperactive piggyBac, currently the most advanced commercially available piggyBac system, and Sleeping beauty 11 (SB11) have been successfully used to develop several CAR T cell therapies that are already in clinical trials (Figure 2). The transposition efficiency of Quantum pBac™ is > 15 x more active than Hyperactive piggyBac and > 10,000 to 40,000 x more active when compared with the transposition efficiency of SB11 (see Figure 2).
The Quantum pBac™system is detailed in Figure 3. It is a two-component system consisting of a “Donor” and a “Helper”, with the donor in a minicircle form to facilitate gene delivery, enhance gene integration, and ensure stable gene expression. The system is safer and more efficient as compared to the Hyperactive piggyBac system (Figure 3).
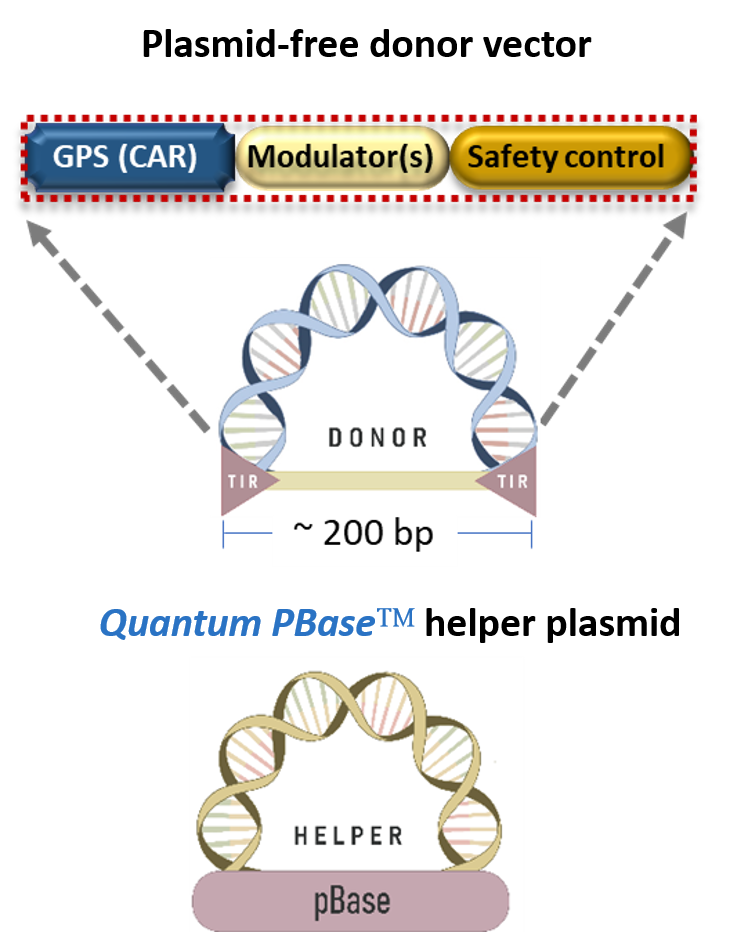
Platform components
A plasmid-Free "Donor" Minicircle DNA
- Comprises a vector with large payload capacity capable of carrying sizable genes of interest
- Contains short terminal inverted repeats (TIRs) that enable safe and effective genetic engineering
- Facilitates gene delivery and improves stability of gene expression
A “Helper” Plasmid
- Promotes integration of genes of interest into the genome of therapeutic cells
- Comprises of Quantum PBase, a highly active and safer piggyBac transposase
Advantages over Hyperactive piggyBac System
- Increased payload capacity & gene delivery rate (smaller donor vector)
- Much less residues (0.1 kb vs hyPB 0.6 kb)
- Less enhancer and/or silencer activities in human T cells
- Potentially safer integration profile
- Increased (> 15 x in human T cells) integration efficiency
Figure 3. Quantum pBac™: Designed to Enhance Therapeutic Gene Integration
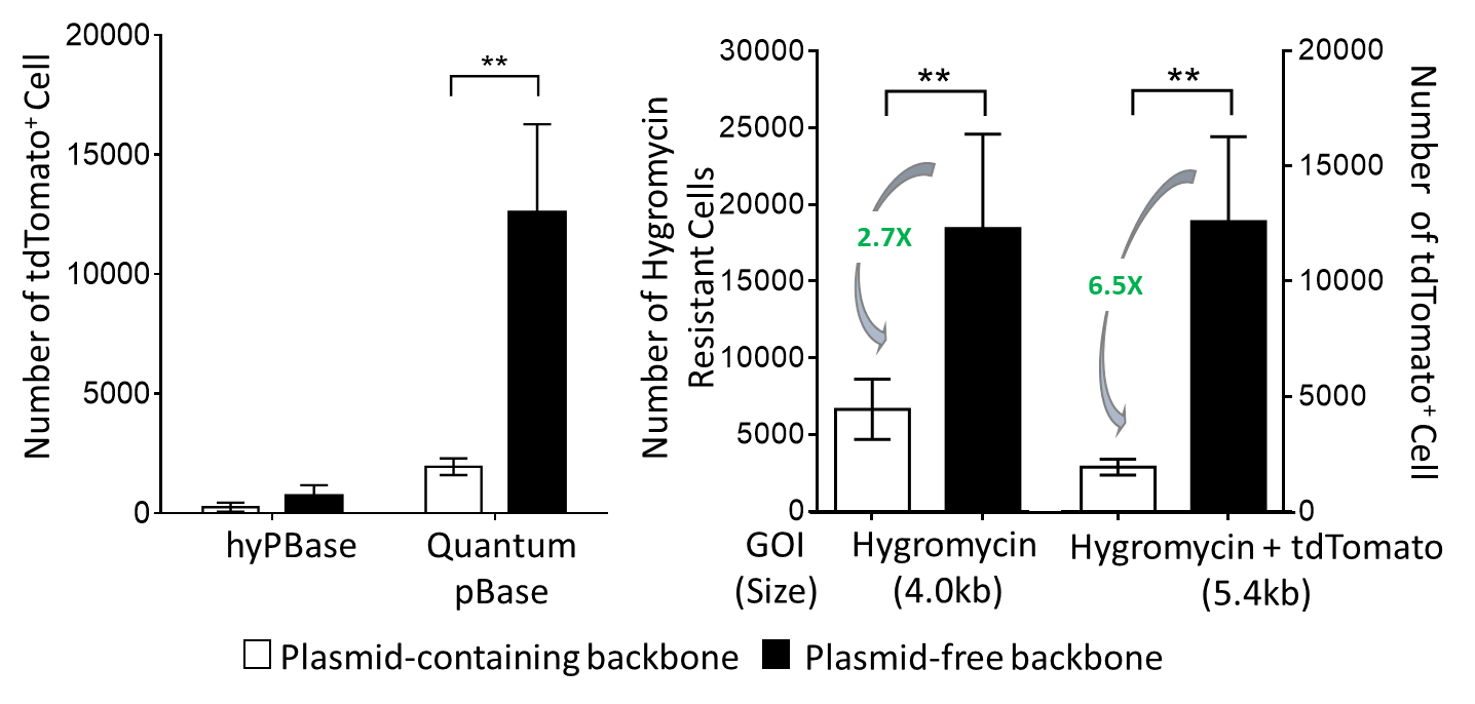
A direct comparison of integration efficiencies between Quantum pBac™ and Hyperactive piggyBac in human T cells shows that Quantum pBac™ is much more efficient (Figure 4).
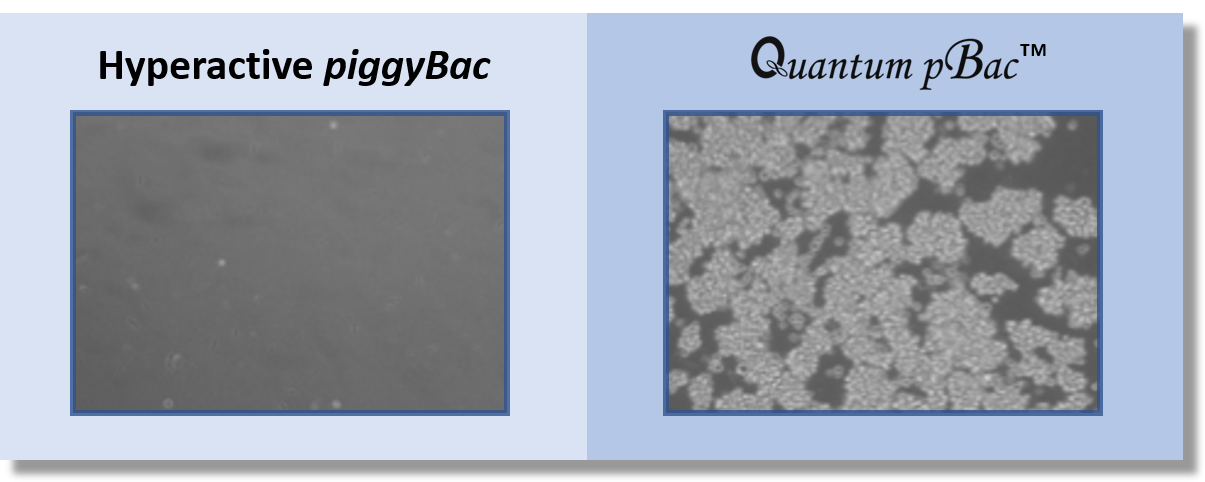
Figure 4. Quantum pBac™ is Superior to Hyperactive PiggyBac.
Jurkat cells were transfected with hygromyci resistance genes carried by either Hyperactive piggyBac (left panel) or Quantum pBac™ (right panel). Images show the number of drug resistant cells remaining following hygromycin selection.
A comparison of transposition efficiency in primary T cells showed that Quantum PBase transposes much more efficiently than hyPBase (see Figure 5). This increase in efficiency was further amplified following removal of backbone plasmid components from the donor vector, and when the vector carried a larger transgene (4.0 kb vs 5.4 kb)